Abstract
Introduction
Diffuse large B-cell lymphoma (DLBCL) presents as limited stage disease in about 25-30% of the patients (pts). It has better 5-year overall survival that advanced stage disease, but is more prone to late relapses, regardless of treatment strategy (Stephens et al, 2016). Based on promising results of radioimmunotherapy consolidation in SWOG S0313 (Persky et al, 2015), and of PET-directed approach by the British Columbia Cancer Agency (Sehn et al, 2011), we designed a PET-directed study to tailor therapy after 3 cycles of R-CHOP. Here, we present the data on safety, response, interim PET, and immunohistochemistry-based cell of origin (COO) analyses.
Methods
SWOG S1001 enrolled non-bulky stage I/II pts with newly diagnosed DLBCL. Pts with mediastinal, HIV-associated, testicular, central nervous system, and indolent lymphoma were excluded. Pts received standard R-CHOP therapy and had an interim PET scan on day 15-18 of cycle 3, which was centrally reviewed. Patients with negative PET scan (Deauville 1-3) proceeded with 1 additional cycle of R-CHOP, while patients with positive PET scan (Deauville 4-5) initiated 36 Gy of IFRT within 35 days of 3rd cycle of R-CHOP, followed by ibritumomab tiuxetan (IFRT-Zevalin) 3-6 weeks after completing IFRT. Final PET scan was performed 12 weeks after treatment completion.
Results
The study completed accrual of 159 pts in June 2016. One pt was upstaged by PET, and 24 pts were ineligible, 20 of them due to incorrect histology based on central pathology review. In 134 eligible pts, median age was 62.2 years, 55.2% had stage I, 17.1% had B symptoms, 14.9% had elevated LDH, and 29.1% had extranodal involvement. Stage modified IPI (Miller et al, 1998) was 0 in 19.4%, 1 in 46.2%, 2 in 31.3%, and 3 in 2.9% of the pts. Baseline PET was negative in 10.4% of the pts since their disease was resected at diagnosis. COO was assessed in 125 eligible pts based on pathology reports using the Hans algorithm - 47.7% were GCB, 25.4% were non-GCB, and in 20.1% there was insufficient information.
Of 132 pts assessible for toxicity after R-CHOP x 3, one died of sepsis, 13 pts had febrile neutropenia, 36 grade (gr) 3/4 neutropenia, 7 gr 3 anemia, 5 gr 3/4 thrombocytopenia, 2 gr 3 lung infection, 3 gr 3 urinary tract infection, and 1 pt had gr 3 peripheral neuropathy.
Of 134 eligible pts, 130 had an interim PET scan centrally reviewed, of which 112 were PET-negative (neg), and 18 were PET-positive (pos). In 4 pts PET was positive due to infection; they were treated as PET-neg pts with additional R-CHOP. One PET-neg pt expired prior to PET-directed therapy, and 2 pts did not receive additional R-CHOP, leaving 113 PET-neg pts assessible for toxicity. One pt died of hypoxia, 1 developed secondary AML, 2 had febrile neutropenia, 12 gr 3/4 neutropenia, 2 gr 3 anemia, 4 gr 3/4 thrombocytopenia. Two PET-pos pts refused radiation. Of 12 PET-pos pts who received IFRT-Zevalin, 2 had gr 3/4 neutropenia, and 3 had gr 3/4 thrombocytopenia.
After 3 cycles of R-CHOP, 116 (87%) pts achieved complete response (CR) and 14 (10%) partial response (PR). Of 115 interim PET-neg pts who received additional R-CHOP 99% obtained CR, with 1 pt having inadequate assessment. Of 12 PET-pos pts 8 (67%) had CR (thus converting from PR after IFRT-Zevalin) and 4 (33%) had PR.
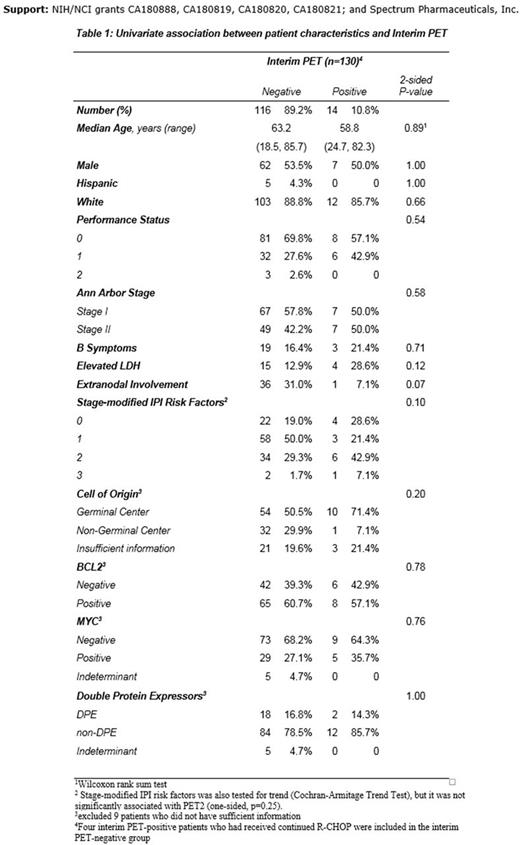
Univariate analyses for interim and final PET scans revealed that interim PET-neg pts were borderline more likely to have extranodal involvement (p=0.07). There were no statistically significant associations, including stage modified IPI and COO (table 1).
Conclusions
SWOG S1001 demostrated feasibility of centralized real-time PET review to direct further therapy in limited stage DLBCL. A high rate of pathology ineligibility, mostly due to concurrent follicular lymphoma, underscores the importance of central pathology review. We show prospectively that limited stage DLBCL is predominantly of GCB origin (65.3% by IHC) and will confirm with a more precise method such as Lymph2Cx. Treatment toxicity was in line with expectations from limited number of R-CHOP cycles. Response rate was high, as expected. The rate of interim PET-positivity was remarkably low (11%), which was at least in part due to classifying Deauville 3 PET scans as negative; relapse rate was monitored every 6 months. While final outcomes need to mature to capture late relapses, this PET-directed therapy approach may allow to target radiation to a minority of patients with limited stage DLBCL.
Persky: Genentech: Consultancy; MorphoSys: Other: Independent Data Monitoring Committee member ; Verastem: Consultancy; Spectrum Pharmaceuticals: Research Funding. Park: Teva: Consultancy, Other: research support; Gilead: Consultancy; Cornerstone: Consultancy, Honoraria; Seattle Genetics: Other: research support; Takeda: Other: research support. Bartlett: Astra Zeneca: Research Funding; Novartis: Research Funding; ImaginAB: Research Funding; Millenium: Research Funding; Pharmacyclics: Research Funding; Janssen: Research Funding; Forty Seven: Research Funding; Immune Design: Research Funding; Bristol-Meyers Squibb: Research Funding; Merck & Co: Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Affimed: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees. Swinnen: Pharmacyclics: Consultancy; Genentech: Consultancy. Barr: Gilead: Consultancy; Novartis: Consultancy; Celgene: Consultancy; Seattle Genetics: Consultancy; Infinity: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding. Leonard: Celgene: Consultancy, Research Funding; Roche: Consultancy. Kahl: Gilead: Consultancy; Celgene: Consultancy; ADC Therapeutics: Research Funding; Seattle Genetics: Consultancy; Genentech: Consultancy. Fisher: Pharmacyclics: Consultancy; F.Hoffmann-LaRoche: Consultancy; Kite: Consultancy; Bayer: Consultancy; Astra Zeneca: Consultancy; Seattle Genetics: Consultancy; Sandoz: Consultancy; Celegene: Consultancy; Genentech: Consultancy. Friedberg: Bayer HealthCare Pharmaceuticals.: Other: Data and Safety Monitoring Board: Bayer HealthCare Pharmaceuticals.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal